Column
Atoms as the Basis for Measuring Both Fleeting Moments and Near-Eternities
 Lake Suigetsu, one of the Mikata Five Lakes in Fukui Prefecture (photo source: Fukui Prefectural Varve Museum)
Lake Suigetsu, one of the Mikata Five Lakes in Fukui Prefecture (photo source: Fukui Prefectural Varve Museum)
Carbon is Present in All Living Things
The opening line of the children's song Sei-Kurabe ("Comparing Heights"), familiar to many in Japan, goes as follows: "The mark on the pillar is my height from the year last before." The song serves as the common human experience, the importance of eating and sleeping well to foster a healthy body. The markings on a wooden pillar serves as a tangible record of a child's development, becoming a cherished part of family history.
All living organisms on Earth rely on organic molecules composed primarily of carbon atoms. These carbon-based molecules are constantly consumed and processed throughout an organism's life. Carbon exists in nature as three primary isotopes: carbon-12 (12C), accounting for approximately 99% of all carbon; carbon-13 (13C), which is slightly heavier than 12C due to an additional neutron; and carbon-14 (14C), a radioactive isotope that decays into nitrogen-14 over time. While 12C and 13C are stable isotopes, 14C is unstable and undergoes radioactive decay.
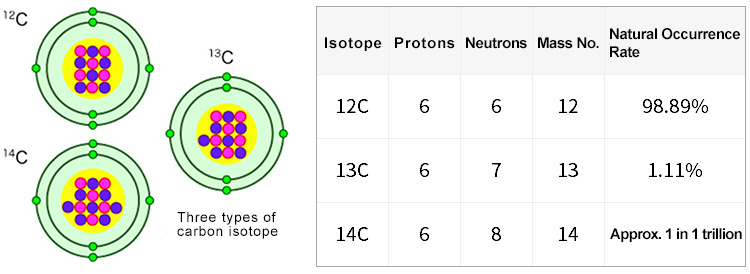 Diagram created based on webpage by the Chronological Research Division, Institute for Space–Earth Environmental Research, Nagoya University
Diagram created based on webpage by the Chronological Research Division, Institute for Space–Earth Environmental Research, Nagoya University
(https://www.nendai.nagoya-u.ac.jp/research/tandetron/radioisotope1.html)
Radioactive decay, the process by which an unstable atomic nucleus spontaneously transforms into a different nucleus, is a probabilistic event. While it is impossible to predict when a specific atom will decay, scientists can determine the "half-life" of a radioactive isotope. The half-life represents the time required for half of the atoms in a given sample to undergo radioactive decay. This period varies significantly among different isotopes. For 14C, the half-life is approximately 5,730 years.
In the Earth's upper atmosphere, cosmic rays interact with nitrogen atoms, transforming them into 14C isotopes. These 14C atoms readily combine with oxygen to form carbon dioxide (CO2), which is then distributed throughout the atmosphere. Living organisms incorporate this atmospheric carbon dioxide into their tissues through photosynthesis and the food chain. Although the abundance of 14C in the atmosphere is minuscule, representing only about one part per trillion of the total carbon, all living organisms contain a measurable amount of 14C at any given time.
For instance, a 70-kilogram (154-pound) human contain only a minuscule amount of 14C, roughly one part per trillion of the total carbon in their body. However, this translates to an astonishing number of 14C atoms – approximately 632 trillion. (This calculation is based on the following: human weight × 18% carbon content in the human body × Avogadro's constant (6.02E23) × 14C abundance rate (1E-12).)
At the time metabolism stops, the 14C content ratio have fixed. This allows scientists to utilize the known half-life of 14C (approximately 5,730 years) to estimate the time elapsed from stop. If a 70-kilogram human body were preserved for 5,730 years, the amount of 14C within the body would be reduced by half. After 11,460 years, only one-quarter of the original 14C would remain, and after 57,300 years, less than 0.1% of the original 14C would be detectable. By measuring the current 14C abundance in a sample and comparing it to the expected level in a living organism, scientists can effectively "reverse-engineer" the time of metabolism stops, a technique known as radiocarbon dating.
Corrections and Calibration: Keys to Improved Measurement Accuracy
Radiocarbon dating is a chronological dating technique that leverages the well-defined half-life of radioactive carbon isotopes. However, accurately determining the age of an organism using this method requires more than simply measuring the decay rate. Various natural factors and environmental influences must be carefully considered and incorporated into the calculations to ensure accurate results.
This scenario is analogous to GNSS positioning and time synchronization calculations. Determining the distance between a satellite and a receiver involves multiplying the signal travel time by the speed of light. However, achieving accurate results necessitates accounting for various factors that delay signal propagation, such as ionospheric plasma and atmospheric precipitation. To further enhance accuracy, it is crucial to incorporate information on the satellite's orbital parameters, discrepancies of satellite's atomic clock time and other relevant variables. In essence, true accuracy hinges on compensating for these hidden factors that are not explicitly considered in the initial distance calculation.
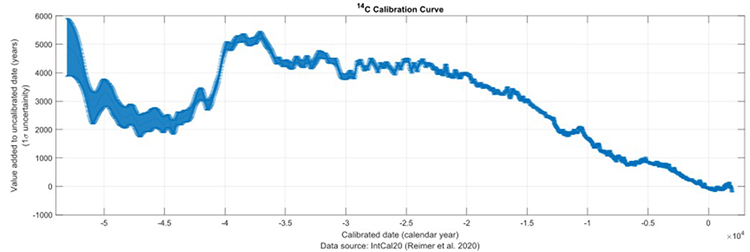 This figure presents a 14C calibration curve based on the IntCal20 model (Reimer et al., 2020). 14C production is influenced by solar activity, leading to variations in atmospheric 14C concentrations. During periods of high solar activity, 14C production increases, causing radiocarbon dates to appear younger than the true calendar age. This calibration curve, derived from independently dated archives, corrects for these fluctuations. The horizontal axis represents calendar age in thousands of years before present (cal kyr BP), while the vertical axis shows the corresponding radiocarbon age. The thickness of the blue line reflects the uncertainty associated with the calibration. This curve provides a valuable tool for accurately dating archaeological and paleoclimatological samples. (Umbricht, 2024, CC 4.0)
This figure presents a 14C calibration curve based on the IntCal20 model (Reimer et al., 2020). 14C production is influenced by solar activity, leading to variations in atmospheric 14C concentrations. During periods of high solar activity, 14C production increases, causing radiocarbon dates to appear younger than the true calendar age. This calibration curve, derived from independently dated archives, corrects for these fluctuations. The horizontal axis represents calendar age in thousands of years before present (cal kyr BP), while the vertical axis shows the corresponding radiocarbon age. The thickness of the blue line reflects the uncertainty associated with the calibration. This curve provides a valuable tool for accurately dating archaeological and paleoclimatological samples. (Umbricht, 2024, CC 4.0)
The IntCal20 calibration curve serves as a fundamental reference for radiocarbon dating, providing a more accurate and precise timeline for events occurring within the last 50,000 years. Constructed through meticulous analysis of various natural archives, including lake sediments, stalagmites, and tree rings, the curve accounts for fluctuations in atmospheric 14C concentrations over time. By aligning measured 14C values from a sample with the IntCal20 curve, researchers can obtain calibrated radiocarbon ages with a typical uncertainty of ±170 years.
 Lake Suigetsu, one of the five Mikata Lakes in Fukui Prefecture, boasts a remarkable 70,000-year record of seasonal changes preserved within 45 meters of well-preserved varved sediment. These layered sediments have played a crucial role in refining radiocarbon dating calibration since the introduction of the IntCal13 model in 2013. The image depicts a section of a core sample extracted from the lakebed. This particular section represents varves deposited between 49,760 ± 164 and 51,345 ± 198 years before the arrival of humans in the Japanese archipelago, according to dating analysis. (Source: Fukui Prefectural Varve Museum, https://varve-museum.pref.fukui.lg.jp/)
Lake Suigetsu, one of the five Mikata Lakes in Fukui Prefecture, boasts a remarkable 70,000-year record of seasonal changes preserved within 45 meters of well-preserved varved sediment. These layered sediments have played a crucial role in refining radiocarbon dating calibration since the introduction of the IntCal13 model in 2013. The image depicts a section of a core sample extracted from the lakebed. This particular section represents varves deposited between 49,760 ± 164 and 51,345 ± 198 years before the arrival of humans in the Japanese archipelago, according to dating analysis. (Source: Fukui Prefectural Varve Museum, https://varve-museum.pref.fukui.lg.jp/)
A Global-Scale Measurement Standard Going Back Fifty Thousand Years
In summary, high-precision radiocarbon dating relies on two key elements: the universally accepted constant of radionuclide half-life and meticulously developed calibration models, the result of extensive scientific research and innovation. I see striking parallels between this technique and GNSS positioning and time synchronization operations:
1. Shared Reliance on Fundamental Principles and Corrections: Both disciplines rely on fundamental physical constants, such as atomic frequencies in GNSS clocks and the half-life of radionuclides in radiocarbon dating. Furthermore, both require the application of correction factors to account for real-world influences, such as radio wave propagation delays in GNSS and variations in atmospheric 14C production in radiocarbon dating.
2.Impact of Solar Activity: Solar activity significantly influences both systems. In radiocarbon dating, solar activity directly affects the production of 14C. Similarly, high solar activity can disrupt GNSS signals, leading to increased positioning and timing errors.
3.Global Timekeeping and Synchronization: Radiocarbon dating provides a global temporal framework by utilizing the ubiquitous presence of 14C in living organisms throughout Earth's history. With a different scales and resolution, GNSS also plays a crucial role in global time synchronization, providing precise time information across the planet.
Very-long-baseline interferometry (VLBI), ancestors of GNSS, leverages celestial phenomena – in this case, radio waves from distant celestial objects – to make precise measurements. Just as radiocarbon dating utilizes the naturally occurring presence of 14C, VLBI capitalizes on the ubiquitous presence of celestial radio sources to provide valuable insights into Earth's rotation, plate tectonics and other scientific facts.
Going Back Further in Time Using Chemical Elements
Radiocarbon dating is a highly accurate method for determining the age of organic materials up to approximately 50,000 years old. For events that occurred much further back in time, such as the dinosaur extinction estimated to have happened 66.05 million years ago, other dating techniques are necessary. This preciseness a significant improvement over the estimates found in many older children's books, was established through a combination of methods, including the analysis of past geomagnetic reversals and radiometric dating using isotopes with much longer half-lives than 14C.
Commonly used nuclides for radiometric dating include potassium-40 (decaying to argon-40 with a half-life of 1.25 billion years) and uranium-238 (decaying to lead through a series of steps with a half-life of 4.50 billion years). These isotopes allow for dating events that occurred hundreds of millions of years ago with a relatively high degree of accuracy, typically within 0.1 to 1 percent.
One particularly long-lived nuclide is rubidium-87, which decays to strontium-87 with a remarkably long half-life of approximately 48.8 billion years – significantly longer than the estimated age of the Universe. The rubidium-strontium (Rb-Sr) dating method, which exploits this extremely slow decay process, is capable of dating geological events that occurred over 10 million years ago. By analyzing the ratio of 87Rb to 87Sr in rocks, while also considering the stable isotope strontium-86, scientists can determine the time at which the rock formed.
Researchers and professionals who rely on precise time synchronization are likely familiar with commercial atomic clocks that utilize rubidium. Next-generation optical lattice clocks are poised to employ strontium instead. It is remarkable to consider that the measurement of time, spanning from fractions of a second as small as one quintillionth (1/100,000,000,000,000,000) to the vast expanse of billions of years, can involve the same chemical element.
This suggests a possible, perhaps even imaginative, connection between the microcosmic and macrocosmic realms, linking the world of fleeting moments with the vastness of cosmic time.
Writer introduction

Mr. Mitsunari Kita Science and technology writer
Born in Ishikawa Prefecture in 1964. Based on his experience in covering industrial technology, cutting-edge technology, and space development, he is passionate about unraveling and conveying difficult topics in an interesting way to people of all ages, from children to senior citizens. From 2009 to 2014, he was a member of the editorial board of "JAXA's," the official magazine of the Japan Aerospace Exploration Agency. Author and co-author of the following books: 『あなたにもミエル化? ~世間のなりたちを工学の視点から~』(幻冬舎mc)、『私たちの「はやぶさ」その時管制室で、彼らは何を思い、どう動いたか』(毎日新聞社)、『東京大学第二工学部70周年記念誌 工学の曙を支えた技術者達』(東京大学生産技術研究所) etc.,
* All registered trademarks used herein are the property of their respective owners.
Pick up
Column
FURUNO Column
-
Common Problems That Affect GPS/GNSS Time Synchronization

-
How to select GPS/GNSS antennas for time synchronization

Column by Mr. Mitsunari Kita (Science and technology writer)
-
The Observation Network Created by the Earthquake Proves Useful for Accurate Timekeeping (Part Two of Two) - A Solution to the "Mr. Higgins Problem" in Space -

-
The Observation Network Created by the Earthquake Proves Useful for Accurate Timekeeping (Part One of Two)

-
FURUNO ELECTRIC Joins Experts From Around the World on a Norwegian Island for Jammertest 2024

-
Unraveling the Mysteries of Venus Based on "Occultation"

-
Atoms as the Basis for Measuring Both Fleeting Moments and Near-Eternities

-
Time Progressing with a Speed Difference of Just 4.4647 Ten-Billionths!

-
Critters Who Revitalize Forests Through the Spreading of Food Caches

-
Small But Significant Variances in Gravity and Time (Part Two of Two)

-
Small But Significant Variances in Gravity and Time (Part One of Two)

-
Why the GT-100 Time-synchronization GNSS Receiver Module is Like Fragrant Soup Curry

-
What Rainbows Can Teach Us About Dual-Band GNSS

-
The Amazing Things That Are Possible With Just a Clock

-
When Subterranean Earth Meets Outer Space

-
Using the TB-1 and GT-100 at a "Multipath Dojo" in the Major Metropolis of Osaka

-
The Disaster-struck Field Time Sync Generator TB-1: True Performance Revealed Through a Lightning Strike

-
Knowing the "Now" of Our Earth Through GNSS

-
The Reason GPS Counts Time in 1.5-second Intervals

-
Similarities Between "On My Count!," the 117 Notification System, and GPS

-
Reliable Clocks Help Us Find a Silver of the Clouds

-
Why Time Synchronization is Vital for Criminal Investigations, Seismograph Measurements and Solar Wind Observation

-
What Was "Cesium" About Cesium Akina?

-
Updating Analog Broadcasting with GNSS Time Synchronization Technology

-
The Long History of One Second (Part II)

-
The Long History of One Second (Part I)

-
A Solo Journey - Three-liter Microsatellite Mission Support via GPS (GNSS) and Satellite Communication -

-
A Solo Journey - The GPS (GNSS) Tracking System That Helped Kenichi Horie Cross the Pacific -

-
The Day After a Superflare - Effects on power and wireless communication infrastructures -





